Jun Yang
Professor of Ophthalmology and Visual Sciences and Adjunct Professor of Neurobiology
Photoreceptors, Hair Cells and Multi-Protein Complex, Treatment of Retinal Degeneration

Molecular Biology Program
Education
B.S. Nankai University, China
Ph.D. University of Massachusetts, Amherst
Research
The research in my laboratory is focused on the genetics of heritable retinal degeneration, cell biology of photoreceptors and gene therapy. We use mouse models and a combination of techniques in molecular and cellular biology, biochemistry, electrophysiology, behavior, and various levels of microscopies.
Retinal degeneration is mainly caused by photoreceptor cell death and retinal pigment epithelium malfunction. It generally includes retinitis pigmentosa and macular degeneration. Although many genes have been identified as responsible for these diseases, their physiological functions and pathogenic mechanisms are not clear. Additionally, many causative genes are still unidentified. Currently, no cure is available.
Disease Mechanism of Usher Syndrome
We investigate the biological functions of genes whose mutations are known to cause human retinal diseases, such as the three known genes involved in Usher syndrome type II, a disease with both vision and hearing loss. We have demonstrated that the encoded proteins of these genes, USH2A, VLGR1 and whirlin, form a multi-protein complex in both photoreceptors and hair cells. The normal localizations and protein amounts of these proteins are dependent on each other in vivo. In photoreceptors, this Usher II protein complex is located at the periciliary membrane complex at the base of the connecting cilium. However, the biological functions of both the Usher II protein complex and the periciliary membrane complex are completely unknown. The outer segment of photoreceptors, the essential cellular compartment for phototransduction, undergoes continuous and rapid renewal, which requires large-scale protein and membrane trafficking from the inner to the outer segment. The Usher II protein complex is currently proposed to be involved in this cellular event. We are now in the process to test this hypothesis.
Cell Biology of Photoreceptors
We are also interested in the cellular processes of intracellular trafficking, structural maintenance, and calcium regulation in photoreceptors. The current projects include 1) the biological functions of the ciliary rootlet, a cytoskeletal structure, in photoreceptors and how its defects cause retinal degeneration; 2) how calcium homeostasis is maintained in photoreceptor synaptic terminals and whether it is involved in retinal degeneration.
Gene Therapy
We study therapeutic treatments for retinal degenerative diseases using mouse models. We have already successfully delivered whirlin into the photoreceptors of whirlin knockout mice via the AAV vectors. We observed correction of molecular defects in this mouse model. Now we continue to study the efficacy of this gene delivery system and how to solve the problem of delivering extremely long genes into the retina.
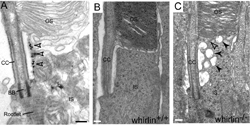
The whirlin protein in photoreceptors. A. Immunoelectron microscopy shows the whirlin protein (black dots, arrows) is localized at the periciliary membrane complex in photoreceptors. B. In wild type photoreceptors, the inner segment (IS) is connected with the outer segment (OS) through the connecting cilium (CC). C. In some of whirlin knockout photoreceptors, the inner segment fuses with the outer segment and vesicles accumulate around the periciliary membrane complex.
References
- Mathur PD, Zou J, Neiswanger G, Zhu D, Wang Y, Almishaal AA, Vashist D, Hammond HK, Park AH, Yang J (2023). Adenylyl cyclase 6 plays a minor role in the mouse inner ear and retina. Scientific Reports, 13, 7075.
- Clark AM, Yu D, Neiswanger G, Zhu D, Zou J, Maschek JA, Burgoyne T, Yang J (2024). Disruption of CFAP418 interaction with lipids causes widespread abnormal membrane-associated cellular processes in retinal degenerations. JCI Insight, 9, e162621.
- Liu X, Lillywhite J, Zhu W, Huang Z, Clark AM, Gosstola N, Maguire CT, Dykxhoorn D, Chen ZY, Yang J (2021). Generation and genetic correction of USH2A c.2299delG mutation in patient-derived induced pluripotent stem cells. Genes, 12, 805.
- Yu D, Zou J, Chen Q, Zhu T, Sui R, Yang J (2020). Structural modeling, mutation analysis, and in vitro expression of usherin, a major protein in inherited retinal degeneration and hearing loss. Comput Struct Biotechnol J, 18, 1363-1382.
- Sharif AS, Yu D, Loertscher S, Austin R, Nguyen K, Mathur PD, Clark AM, Zou J, Lobanova ES, Arshavsky VY, Yang J (2018). C8ORF37 is required for photoreceptor outer segment disc morphogenesis by maintaining outer segment membrane protein homeostasis. J Neurosci, 38(13), 3160-3176.
- Zou J, Chen Q, Almishaal A, Mathur PD, Zheng T, Tian C, Zheng QY, Yang J (2017). The roles of USH1 proteins and PDZ domain-containing USH proteins in USH2 complex integrity during hair cell development. Human Molecular Genetics 26(3), 624-636.
- Mathur P, Zou J, Zheng T, Almishaal A, Wang Y, Chen Q, Wang L, Vashist D, Brown S, Park A, Yang J (2015). Distinct expression and function of whirlin isoforms in the inner ear and retina: an insight into pathogenesis of USH2D and DFNB31. Human Molecular Genetics 24(21), 6213-6228.
- Zou J, Mathur P, Zheng T, Wang Y, Almishaal A, Park A, Yang J (2015). Individual USH2 proteins make distinct contributions to the ankle link complex during development of the mouse cochlear stereociliary bundle. Human Molecular Genetics 24(24), 6944-6957.
- Mathur P, Vijayakumar S, Vashist D, Jones SM, Jones TA, Yang J (2015). A study of whirlin isoforms in the mouse vestibular system suggests potential vestibular dysfunction in DFNB31-deficient patients. Human Molecular Genetics 24(24), 7017-7030.
- Ciardo MG, Andres-Borderia A, Cuesta N, Valente P, Camprubi-Robles M, Yang J, Planells-Cases R, Ferrer-Montiel A (2016). Whirlin increases TRPV1 channel expression and cellular stability. Biochimica et Biophysica Acta 1863(1), 115-127.
- Chen Q, Zou J, Shen Z, Zhang W, Yang J (2014). Whirlin and PDZ domain containing 7 (PDZD7) proteins are both required to form the quaternary protein complex associated with Usher syndrome type 2. Journal of Biological Chemistry289(52), 36070-36088.
- Mathur P, Yang J (2014). Usher syndrome: hearing loss, retinal degeneration and associated abnormalities. [Review]. BBA - Molecular Basis of Disease 1852(3), 406-420.
- Zou J, Zheng T, Ren C, Askew C, Liu XP, Pan B, Holt JR, Wang Y, Yang J (2014). Deletion of PDZD7 disrupts the Usher syndrome type 2 protein complex in cochlear hair cells and causes hearing loss in mice. Human Molecular Genetics 23(9), 2374-2390.
- Zou J , Lee A , Yang J (2012). The expression of whirlin and Cav1.3α₁ is mutually independent in photoreceptors. Vision Research 75, 53-59.
- Yang J (2012). Usher Syndrome: Genes, Proteins, Models, Molecular Mechanisms, and Therapies. In Sadaf Naz (Ed.), Hearing Loss (pp. 293-328). Rijeka: Intech, ISBN: 978-953-51-0366-0.
- Yang J, Wang L, Song H, Sokolov M (2012). Current Understanding of Usher Syndrome Type II. [Review]. Frontiers in Bioscience 17, 1165-1183.
- Wang L, Zou J, Shen Z, Song E, Yang J (2012). Whirlin interacts with espin and modulates its actin-regulatory function: an insight into the mechanism of Usher syndrome type II. Human Molecular Genetics 21(3), 692-710.
- Zuo JH, Luo L, Shen Z, Choido V, Ambati BK, Hauswirth WW, Yang J (2011). Whirlin replacement restores the formation of the USH2 protein complex in whirlin knockout photoreceptors. Investigative Ophthalmology & Visual Science 52(5), 2343-2351.
- Yang J, Liu X, Zhao Y, Adamian M, Pawlyk B, Sun X, McMillan DR, Liberman MC, Li T (2010). Ablation of whirlin long isoform disrupts the USH2 protein complex and causes vision and hearing loss. PLoS Genetics 6(5), e10000955.
- Yang J, Pawlyk B, Wen X, Adamian M, Soloviev M, Michaud N, Zhao Y, Sandberg MA, Makino CL, Li T (2007). Mpp4 is required for proper localization of plasma membrane calcium ATPases and maintenance of calcium homeostasis at the rod photoreceptor synaptic terminals. Human Molecular Genetics 16(9), 1017-1029.
- Yang J, Li T (2006). Focus on molecules: Rootletin. Experimental Eye Research 83(1), 1-2.
- Yang J, Adamian M, Li T (2006). Rootletin interacts with C-Nap1 and may function as a physical linker between the pair of centrioles/basal bodies in cells. Molecular Biology of the Cell 17(2), 1033-1040.
- Yang J, Li T (2005). The ciliary rootlet interacts with kinesin light chains and may provide a scaffold for kinesin-1 vesicular cargos. Experimental Cell Research 309(2), 379-389.
- Yang J, Gao J, Adamian M, Wen X, Pawlyk B, Zhang L, Sanderson MJ, Zuo J, Makino CL, Li T (2005). The ciliary rootlet maintains long-term stability of the sensory cilia. Molecular and Cellular Biology 25(10), 4129-4137.
- Yang J, Liu X, Yue G, Adamian M, Bulgakov O, Li T (2002). Rootletin, a novel coiled-coil protein, is a structural component of the ciliary rootlet. Journal of Cell Biology 159(3), 431-440.
