Saveez Saffarian
Associate Professor of Physics and Astronomy and Adjunct Associate Professor of Biological Sciences
Enveloped Virus Budding

Biological Chemistry Program
Education
B.S. Sharif University of Technology, Iran
Ph.D. Washington University, St Louis
Research
My lab is focused on development of microscopy techniques which will merge live imaging and high resolution microscopy, visualizing many of the essential molecular mechanisms critical for the function of retroviruses specifically HIV as well as negative strand RNA viruses specifically VSV. Our current microscopy focus is to extend capabilities of the iPALM microscopy for single molecule tracking and high resolution imaging of virological samples. We are especially keen in applying the fluctuations based methodologies as well as high resolution imaging to better understand transport and molecular mechanisms driving the biological mechanisms in VSV replication and HIV budding and maturation.
HIV Budding and Maturation
Our long-term goal is to gain a molecular understanding of the machinery that regulates the release of infectious HIV virions from infected cells. Newly released HIV virions are immature and non-infectious. The immature HIV lattice is composed of ~120 copies of Gag-Pol along with ~2000 copies of Gag. The immature virion incorporates the HIV protease as a monomer within the Gag-Pol precursor. During budding, HIV recruits members of the Endosomal Sorting Complexes Required for Transport to help catalyze its release from host cells. It is believed that sometime after release of virions, HIV protease monomers are released from the confines of the Gag-Pol proteins and form active protease dimers which in turn drive the transformation of the immature lattice to infectious mature HIV core. During the past couple of years, we evaluated the exact role of early Endosomal Sorting Complexes Required for Transport (ESCRT), ALIX and TSG101, in HIV budding. We found that these early ESCRTs play a major role in a molecular race between virion budding and activation of HIV protease which is essential for virion maturation and infectivity. Currently we are interested in understanding the exact molecular mechanism by which ESCRTs catalyze the release of HIV virions. We are also interested in understanding the molecular mechanism that drives the process of maturation specifically initiation of protease release from Gag-Pol molecules and the relation of these molecular events to virion release.
Dynamics of RNA Dependent RNA Polymerases during Replication of Negative Strand RNA Viruses
Negative strand non-segmented (NNS) RNA viruses include potent human and animal pathogens, e.g. Ebola, measles and vesicular stomatitis virus (VSV). To transcribe and replicate their genome, these viruses package multiple copies of an RNA dependent RNA polymerase (RdRP), within each virion. While the virion morphology varies among NNS RNA viruses, the basic mechanism of transcription and replication is shared. Specifically, the genome template in NNS RNA viruses consists of a single molecule of negative sense RNA, typically encoding more than 5 genes and fully encapsidated with the nucleoprotein N (N-RNA). RdRP polymerases transcribe the genome sequentially by initiating transcription at or near the 3' end and moving toward the 5' end. While the N-RNA bound with RdRPs is the deadly engine within many major human pathogens, it’s transcription mechanism is distinct from cellular DNA based RNA polymerases and remains poorly understood. We are focused on establishing a fundamental understanding of the RdRP transcription and open the door to its further applications.
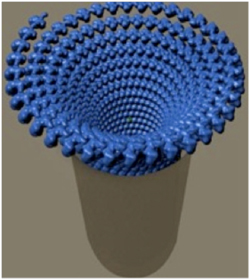
Artistic rendering of Vesicular Stomatitis Virus during RNA capture at the plasma membrane. For simplicity only the N molecule is shown. The illustrated packing within the virion is consistent with Cryo EM studies of VSV. The extended spiral outside the formed virion is an artistic expression. It is not clear for how long the RNA would retain its spiral twist in the cytoplasm.
References
- Saffarian S.Application of Advanced Light Microscopy to the Study of HIV and Its Interactions with the Host. Viruses. 2021; 13
- Sharma A., Preece B., Swann H., Fan X., McKenney R. J., Ori-McKenney K. M., Saffarian S., Vershinin M. D. Structural stability of SARS-CoV-2 virus like particles degrades with temperature. Biochemical and Biophysical Research Communications. 2021;534, 343–346.
- Swann H., Sharma A., Preece B., Peterson A., Eldridge C., Belnap D. M., Vershinin M., Saffarian S. Minimal system for assembly of SARS-CoV-2 virus like particles. Scientific Reports. 2020; 10, 21877.
- Gupta S., Bendjennat M., Saffarian S. Abrogating ALIX Interactions Results in Stuttering of the ESCRT Machinery. Viruses 2020; 12(9): 1032
- Saha I., Saffarian S. Dynamics of the HIV Gag Lattice Detected by Localization Correlation Analysis and Time-Lapse iPALM. Biophysical Journal. 2020; 119(3):581-592.
- Gupta S., Bromley J., Saffarian S. High-speed imaging of ESCRT recruitment and dynamics during HIV virus like particle budding. PLOS One. 2020; 15(9):e0237268.
- Saha I., Saffarian S. Interferometric Fluorescent Cross Correlation Spectroscopy. PLOS One. 2019; 14(12):e0225797
- Gupta S., Bendjennat M., Saffarian S. Abrogating ALIX interactions results in stuttering of the ESCRT machinery. BiorXive. 2019.
- Bendjennat M., Saffarian S. Fluorescent Protein Inserts in between NC and SP2 Are Tolerated for Assembly, Release and Maturation of HIV with Limited Infectivity. Viruses. 2019; 11(11), 973
- Peterson M, Jamali S, Daum R, Bendjennat M, Saffarian S. Correlative iPALM and SEM resolves virus cavity and Gag lattice defects in HIV virions. European Biophysical Journal. 2019
- Bendjennat M, Saffarian S. The Race against Protease Activation Defines the Role of ESCRTs in HIV Budding. PLoS Pathogens. 2016;12(6):e1005657. PMCID: PMC4900648
- Tang X, Bendjennat M, Saffarian S. Vesicular Stomatitis Virus Polymerase's Strong Affinity to Its Template Suggests Exotic Transcription Models. PLoS Comput Biol. 2014;10(12):e1004004.
- Ku P-I, Bendjennat M, Ballew J, Landesman MB, Saffarian S. ALIX Is Recruited Temporarily into HIV-1 Budding Sites at the End of Gag Assembly. PLoS ONE. 2014;9(5):e96950. PMCID: PMID: 24834918.
- Hodges J.A., Saffarian S. Sample Preparation for Single Virion Atomic Force Microscopy and Super-resolution Fluorescence Imaging. JoVE. 2014; (83):e51366.
- Ku P-I, Miller Anna K, Ballew J, Sandrin V, Adler Frederick R, Saffarian S. Identification of Pauses during Formation of HIV-1 Virus Like Particles. Biophysical Journal. 2013;105(10):2262-72. PMCID: PMID: 24268138.
- Hodges J, Tang X, Landesman MB, Ruedas JB, Ghimire A, Gudheti MV, et al. Asymmetric packaging of polymerases within vesicular stomatitis virus. Biochemical and Biophysical Research Communications. 2013;440(2):271-6.
- Collier I. E., Legant W., Marmer B., Lubman O., Saffarian S., Wakatsuki T., Elson E., Goldberg G I. Diffusion of MMPs on the Surface of Collagen Fibrils: The Mobile Cell Surface – Collagen Substratum Interface. PLoS ONE. 2011;6(9):e24029
- Boucrot E, Saffarian S, Zhang R, Kirchhausen T. Roles of AP-2 in Clathrin-Mediated Endocytosis. PLoS ONE. 2010;5(5):e10597.
- Thiery J., Keefe D., Saffarian S., Martinvalet D., Walch M., Boucrot E., Kirchhausen T., Lieberman J. Perforin activates clathrin- and dynamin-dependent endocytosis, which is required for plasma membrane repair and delivery of granzyme B for granzyme-mediated apoptosis. Blood. 2010;115(8):1582-93
- Saffarian S, Cocucci E, Kirchhausen T. Distinct Dynamics of Endocytic Clathrin-Coated Pits and Coated Plaques. PLoS Biol. 2009;7(9):e1000191. PMCID: PMID: 19809571.
- Saffarian S., Kirchhausen T. Differential Evanescence Nanometry: Live Cell Fluorescence Measurements with 10 nm Axial Resolution on the Plasma Membrane. Biophysical Journal. 2008; 94(6)2333-42. PMCID: PMID: 17993495.
- Saffarian S, Li Y, Elson EL, Pike LJ. Oligomerization of the EGF Receptor Investigated by Live Cell Fluorescence Intensity Distribution Analysis. Biophysical Journal. 2007;93(3):1021-31. PMCID: PMID: 17496034.
- Boucrot E., Saffarian S., Massol R., Kirchhausen T., Ehrlich M. Role of lipids and actin in the formation of clathrin-coated pits. Experimental CellResearch 2006; 312(20): 4036-4048
- Saffarian S., Qian H., Collier I. E., Elson E. L., Goldberg G. Powering a Burnt Bridges Ratchet: A Model for an Extracellular Motor Driven by Proteolysis of Collagen. Phys Rev E. 2006; 73: 041909
- Chattopadhyay K, Saffarian S, Elson EL, Frieden C. Measuring Unfolding of Proteins in the Presence of Denaturant Using Fluorescence Correlation Spectroscopy. Biophysical Journal. 2005;88(2):1413-22. PMCID: PMID: 15556973.
- Saffarian S., Collier I.E., Marmer B.L., Elson E.L., Goldberg G. Interstitial Collagenase is an ATP - independent Molecular Motor Driven by Proteolysis of Collagen. 2004; Science 306: 108-111
- Saffarian S, Collier IE, Marmer BL, Elson EL, Goldberg G. Interstitial Collagenase Is a Brownian Ratchet Driven by Proteolysis of Collagen. Science. 2004;306(5693):108-11. PMCID: PMID: 15459390.
- Saffarian S, Elson EL. Statistical Analysis of Fluorescence Correlation Spectroscopy: The Standard Deviation and Bias. Biophysical Journal. 2003;84(3):2030-42.
- Chattopadhyay, Krishnananda, Saffarian S., Elson E.L., Frieden C. Measurement of Microsecond Dynamic Motion in the Intestinal Fatty Acid Binding Protein using Fluorescence Correlation Spectroscopy. PNAS. 2002; 99: 14171-14176
- Qian, H., Saffarian S., Elson E.L. Concentration fluctuations in a mesoscopic oscillating chemical reaction system. PNAS. 2002; 99: 10376-10381
- Collier I.E., Saffarian S., Marmer B.L., Elson E.L., Goldberg G. Substrate Recognition by Gelatinase A: The C-Terminal Domain Facilitates Surface Diffusion. Biophysical Journal. 2001; 81: 2370-2377
