Vicente Planelles
Professor of Microbiology and Immunology
Pathogenesis of HIV-1

Molecular Biology Program
Education
B.S. Universidad Complutense, Spain
Ph.D. University of California, Davis
Research
Our laboratory has a general interest in the pathogenesis by HIV-1 and other primate lentiviruses. Viral pathogenesis results from the interaction of the viral genes/proteins with those of the host cell. Therefore, a profound knowledge of cellular biology is absolutely required if one wants to understand viral pathogenesis. Because of that, we have acquired and developed tools and knowledge in various areas of cell biology including cell cycle, apoptosis, DNA damage, the ubiquitin/proteasome system, T-cell signaling pathways, and others. Today, our research focuses on two specific areas of HIV pathogenesis:
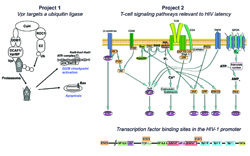
- The role of Vpr in viral pathogenesis. Vpr is a multifunctional protein of only 96 amino acid residues. In the context of HIV, Vpr induces cell cycle arrest in G2, followed by apoptosis. Recent investigations from several laboratories have identified a ubiquitin ligase enzyme with the composition Cul4a/DDB1/DCAF1 as the mediator of both of the above cytopathic effects, and perhaps other as yet unknown effects. Vpr directly docks onto the E3 ligase via its substrate specificity module, DCAF1, and presumably redirects the specificity toward a non-cognate substrate. Read more in (DeHart and Planelles, J. Virol 2007, 82:1066).
- Induction of HIV-1 latency in memory CD4+ T cells, and reactivation of latent viruses. The advent of highly active antiretroviral therapy (HAART) has revealed that, even in the patients who best respond to therapy, virus eradication fails to occur. We now know that there are multiple viral reservoirs that are refractory to inhibition by antiretrovirals. A reservoir that is particularly long lived is the CD4+ memory T-cell subset. HIV-1 latency in this subset appears to be defined by a lack of viral gene expression. In our lab, we have developed a novel assay that recapitulates the transition of CD4+ cells from the activated to the memory stages, and allows for very efficient establishment of latency by HIV-1. Using this system, we are in the process of dissecting the signaling pathways and transcription factors that facilitate or impede both latency and reactivation. Read more in (Bosque and Planelles, Blood 2009, 113:58).
References
-
Szaniawski MA, Spivak AM, Bosque A, Planelles V (2019) Sex Influences SAMHD1 Activity and Susceptibility to Human Immunodeficiency Virus-1 in Primary Human Macrophages. J Infect Dis. 219(5):777-785. doi: 10.1093/infdis/jiy583.
-
Saayman, S. M., D. C. Lazar, T. A. Scott, J. R. Hart, M. Takahashi, J. C. Burnett, V. Planelles, K. V. Morris, M. S. Weinberg (2015) Potent and Targeted Activation of Latent HIV-1 Using the CRISPR/dCas9 Activator Complex. Mol Ther. 24(3):488-98.
-
Spivak, A. M., and V. Planelles. (2016) HIV-1 Eradication: Early Trials (and Tribulations). Trends Mol Med 22(1):10-27.
-
Pache, L., M. S. Dutra, A. M. Spivak, J. M. Marlett, J. P. Murry, Y. Hwang, A. M. Maestre, L. Manganaro, M. Vamos, P. Teriete, L. J. Martins, R. Konig, V. Simon, A. Bosque, A. Fernandez-Sesma, N. D. Cosford, F. D. Bushman, J. A. Young, V. Planelles, and S. K. Chanda. (2015) BIRC2/cIAP1 Is a Negative Regulator of HIV-1 Transcription and Can Be Targeted by Smac Mimetics to Promote Reversal of Viral Latency. Cell Host Microbe 18(3):345-53.
-
Ramirez, P. W., A. B. DePaula-Silva, M. Szaniawski, E. Barker, A. Bosque, and V. Planelles. (2015) HIV-1 Vpu utilizes both cullin-RING ligase (CRL) dependent and independent mechanisms to downmodulate host proteins. Retrovirology 12:65.
-
Ramirez, P. W., M. Famiglietti, B. Sowrirajan, A. B. DePaula-Silva, C. Rodesch, E. Barker, A. Bosque, and V. Planelles. (2014) Downmodulation of CCR7 by HIV-1 Vpu Results in Impaired Migration and Chemotactic Signaling within CD4+ T Cells. Cell Reports 7(6):2019-30.
-
Sherrill-Mix, S., M. K. Lewinski, M. Famiglietti, A. Bosque, N. Malani, K. E. Ocwieja, C. C. Berry, D. Looney, L. Shan, L. M. Agosto, M. J. Pace, R. F. Siliciano, U. O'Doherty, J. Guatelli, V. Planelles, and F. D. Bushman. (2013) HIV latency and integration site placement in five cell-based models. Retrovirology 10:90.
-
Budhiraja, S., M. Famiglietti, A. Bosque, V. Planelles, and A. P. Rice. (2013) Cyclin T1 and CDK9 T-loop phosphorylation are downregulated during establishment of HIV-1 latency in primary resting memory CD4+ T cells. J Virol. 87(2):1211-20.
-
Spina, C. A., et al. (2013) An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathog 9(12):e1003834.
-
Planelles, V. (2012) SAMHD1 Joins the Red Queen’s Court. Cell Host & Microbe 11(2):103-5.
-
Bosque, A., M. Famiglietti, A. S. Weyrich, C. Goulston, and V. Planelles. (2011) Homeostatic Proliferation Fails to Efficiently Reactivate HIV-1 Latently Infected Central Memory CD4+ T Cells. PLoS Pathog. 7(10):e1002288.
