Katsu Funai
Associate Professor of Nutrition and Integrative Physiology and Adjunct Associate Professor of Biochemistry
Lipids, Mitochondria, Bioenergetics, Metabolic Disease

Molecular Biology Program
Biological Chemistry Program
Education
B.S./M.S. Boston University
Ph.D. University of Michigan
Research
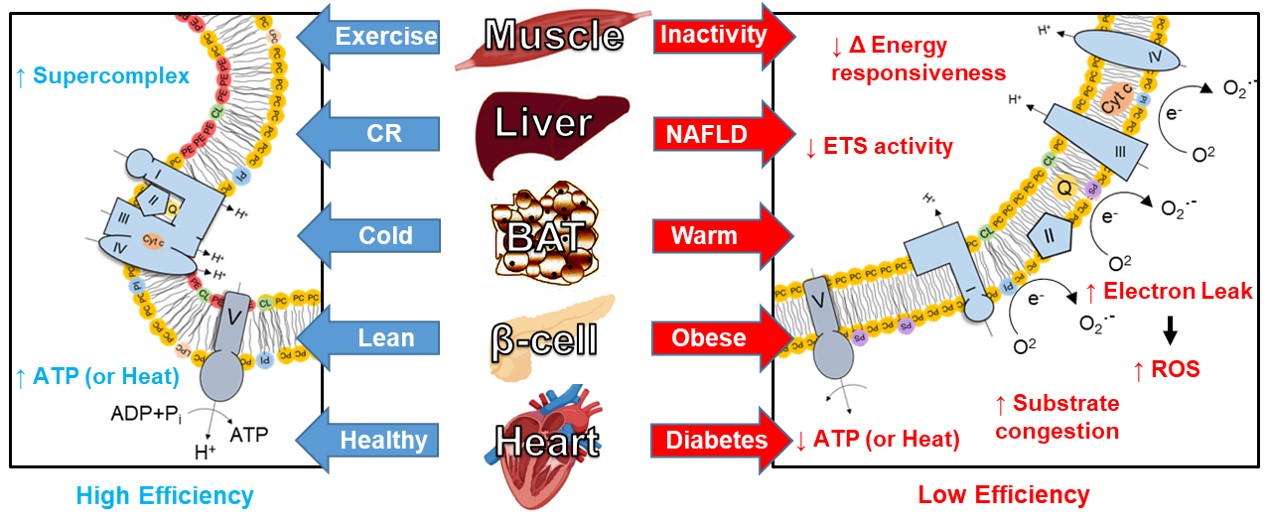
The Funai laboratory is interested in the intracellular fate of lipids into membrane phospholipids and how they affect cellular energetics. In particular, mitochondrial membrane lipid composition becomes robustly altered upon metabolic insults in multiple tissues. Genetic disruptions or enhancements in pathways of mitochondrial phospholipid biosynthesis are sufficient to recapitulate some of the physiological/pathological phenotypes induced by such metabolic insults. Human mutations in the enzymes of mitochondrial phospholipid biosynthesis cause severe mitochondrial defects and are detrimental to health.
As a postdoctoral fellow in Clay Semenkovich’s lab, Katsu developed a deep appreciation for subcellular lipid compartmentalization and decided to pursue this topic when he started his laboratory in 2013. We first characterized the relationship between mitochondrial lipidome and bioenergetics in skeletal muscle (Heden et al., Sci Adv, 2019). We found that exercise and physical inactivity robustly alter mitochondrial phosphatidylethanolamine (PE) and described the role of this lipid in improving OXPHOS efficiency and driving some of the physiological phenotypes associated with exercise or inactivity. Leveraging this strategy, we are now beginning to implicate mitochondrial lipids in other tissues such as adipose tissues, liver, pancreatic β cells, proximal tubules in the kidney, etc. (Verkerke et al., Nat Metab, 2019; Johnson et al., Mol Metab, 2020; Ferrara et al., biorχiv, 2022; Siripoksup et al., biorχiv, 2022; Johnson et al., Sci Adv, 2023). Together, we hypothesize that mitochondrial membrane lipids represent a common, fundamental mechanism by which OXPHOS efficiency is altered to trigger metabolic diseases. In addition to our studies in mitochondrial lipids, we have also studied ER phospholipids, plasma membrane phospholipids, as well as lipid peroxidation. Our lab is currently funded by grants from the National Institutes of Health and American Heart Association
References (Selected Publications)
- Decker ST, Funai K. Mitochondrial membrane lipid in the regulation of bioenergetic flux. Cell Metabolism. In Press.
- Siripoksup P, Cao G, Cluntun AA, Maschek JA, Pearce Q, Lang MJ, Jeong MY, Eshima H, Ferrara PJ, Opurum PC, Mahmassani ZS, Peterlin AD, Watanabe S, Walsh MA, Taylor EB, Cox JE, Drummond MJ, Rutter J, Funai K. Sedentary behavior in mice induces metabolic inflexibility by suppressing skeletal muscle pyruvate metabolism. J Clin Invest. 134(11):e167371, 2024.
- Eshima H, Shahtout JL, Siripoksup P, Pearson MJ, Mahmassani ZS, Ferrara PJ, Lyons AW, Maschek JA, Peterlin AD, Verkerke ARP, Johnson JM, Salcedo A, Petrocelli JJ, Miranda ER, Anderson EJ, Boudina S, Ran Q, Cox JE, Drummond MJ, Funai K. Lipid hydroperoxides promote sarcopenia through carbonyl stress. eLife. 12:e85289, 2023.
- Ferrara PJ, Lang MJ, Johnson JM, Watanabe S, McLaughlin KL, Maschek JA, Verkerke ARP, Siripoksup P, Chaix A, Cox JE, Fisher-Wellman KH, Funai K. Weight loss improves skeletal muscle mitochondrial energy efficiency. Life Metabolism. 2(2):load014, 2023.
- Johnson JM, Peterlin AD, Balderas E, Sustarsic EG, Maschek JA, Lang MJ, Jara-Ramos A, Panic V, Morgan JT, Villanueva CJ, Sanchez A, Rutter J, Lodhi IJ, Cox JE, Fisher-Wellman KH, Chaudhuri D, Gerhart-Hines Z, Funai K. Mitochondrial phosphatidylethanolamine directly regulates UCP1 to promote brown adipose thermogenesis. Science Advances. 9(8):eade7864, 2023.
- Miranda ER, Shahtout JL, Funai K. Chicken or Egg? Mitochondrial phospholipids and oxidative stress in disuse-induced skeletal muscle atrophy. Antioxidants & Redox Signaling. 38(4-6):338-351, 2023.
- Miranda ER, Funai K. Suppression of de novo sphingolipid biosynthesis mitigates sarcopenia. Nature Aging. 2, 1088-1089, 2022.
-
Ferrara PJ, Rong X, Maschek JA, Verkerke ARP, Siripoksup P, Song H, Green TD, Krishnan KC, Johnson JM, Turk J, Houmard JA, Lusis AJ, Drummond MJ, McClung JM, Cox JE, Shaikh SR, Tontonoz P, Holland WL, Funai K. Lysophospholipid acylation modulates plasma membrane lipid organization and insulin sensitivity in skeletal muscle. J Clin Invest. 131(8): e135963, 2021.
-
Funai K, Summers SA, Rutter J. Reign in the Membrane: How common lipids govern mitochondrial function. Current Opinion in Cell Biology. 63:162-173, 2020.
- Johnson JM, Verkerke ARP, Maschek JA, Ferrara PJ, Lin C, Kew KA, Neufer PD, Lodhi IJ, Cox JE, Funai K. Alternative splicing of UCP1 by non-cell autonomous action of PEMT. Molecular Metabolism. 31(1):55-66, 2020.
- Verkerke ARP, Ferrara PJ, Lin C, Johnson JM, Ryan TE, Maschek JA, Eshima H, Paran CW, Laing BT, Siripoksup P, Tippetts TS, Wentzler EJ, Huang H, Spangenburg EE, Brault JJ, Villanueva CJ, Summers SA, Holland WL, Cox JE, Vance DE, Neufer PD, Funai K. Phospholipid methylation regulates muscle metabolic rate through Ca2+ transport efficiency. Nature Metabolism. 1(9):876-885, 2019. [Selected for Cover]
- Heden TD, Johnson JM, Ferrara PJ, Eshima H, Verkerke ARP, Wentzler EJ, Siripoksup P, Narowski TM, Coleman CB, Lin CT, Ryan TE, Reidy PT, de Castro Bras LE, Karner CM, Burant CF, Maschek JA, Cox JE, Mashek DG, Kardon G, Boudina S, Zeczycki TN, Rutter J, Shaikh SR, Vance JE, Drummond MJ, Neufer PD, Funai K. Mitochondrial PE potentiates respiratory enzymes to amplify skeletal muscle aerobic capacity. Science Advances. 5(9):eaax8352, 2019..
- Park H, He A, Tan M, Johnson JM, Dean JM, Pietka TA, Chen Y, Zhang X, Hsu FF, Razani B, Funai K, Lodhi IJ. Peroxisome-derived lipids regulate adipose thermogenesis by mediating cold-induced mitochondrial fission. J Clin Invest. 129(2):694-711, 2019.
- Pennington ER, Funai K, Brown DA, Shaikh SR. The role of cardiolipin concentration and acyl-chain composition on mitochondrial inner membrane molecular organization and function. Biochim Biophys Acta. 1864(7):1039-1052, 2019.
- Anderson EJ, Vistoli G, Katunga, LA, Funai K, Regazzoni L, Monroe TB, Gilardoni E, Cannizzaro L, Colzani M, De Maddis D, Rossoni G, Canevotti R, Gagilardi S, Carini M, Aldini G. A carnosine analog mitigates metabolic disorders of obesity by reducing carbonyl stress. J Clin Invest. 128(12):5280-5293, 2018.
- Johnson JM, Ferrara PJ, Verkerke ARP, Coleman CB, Wentzler EJ, Neufer PD, Kew KA, de Castro Brás LE, Funai K. Targeted overexpression of catalase to mitochondria does not prevent cardioskeletal myopathy in Barth syndrome. J Mol Cell Cardiol. 121(8):94-102, 2018.
- Ferrara PJ, Verkerke ARP, Brault JJ, Funai K. Hypothermia decreases oxygen cost for ex vivo contraction in mouse skeletal muscle. Med Sci Sports Exerc. 50(10):2015-2023, 2018.
- Heden TD, Neufer PD, Funai K. Looking beyond structure: membrane phospholipids of skeletal muscle mitochondria. Trends Endocrinol Metab. 27(8):553-62, 2016.

