Rodney Stewart
Associate Professor of Oncological Sciences
Zebrafish Cancer Models, Childhood Cancers, Brain Tumors, Embryology, Live-Imaging, Genetics, Genomics and Epigenetics

Molecular Biology Program
Education
B.S. University of Melbourne, Australia
Ph.D. Yale University
Research
Research in our laboratory is focused on mechanisms driving pediatric brain cancer, the leading cause of cancer-related deaths in children. During brain tumor development, genomic changes often rewire, or hijack, normal embryonic programs of cell proliferation, survival and migration to promote tumor growth, invasion and chemoresistance. Targeting embryonic mechanisms in brain tumor cells is therefore expected to lead to new treatments and improved patient outcomes. However, to do this, a deeper understanding of the rate-limiting mechanisms controlling normal embryonic development are needed, as well as generation of pre-clinical models to identify and test such treatments. To tackle this difficult problem, our lab uses embryology, genetics, genomics and cancer modeling approaches. We use the zebrafish model in our studies due to its superior genetic and imaging capabilities and human cells (cultured and primary cells) to identify conserved mechanisms. Current projects in the lab include:
- Generating new animal models of pediatric brain tumors. Recent advances in genomics have revolutionized the identification of pediatric brain tumor subtypes and identified many potential genetic drivers. The challenge now is to determine if one or more genomic changes associated with specific pediatric brain tumors can actually drive tumorigenesis and/or invasion. We use transgenic approaches in zebrafish, combined with comparative onco-genomics, to identify cell of origin and novel genetic/genomic drivers of newly defined brain tumor entities. Current projects include generation of high-risk medulloblastoma subtypes (MYC- and SHH-driven) and incurable glioma subtypes, such as Diffuse Pontine Glioma (DIPG).
- Identifying genetic/epigenetic regulators of the Epithelial-to-Mesenchymal Transition (EMT). EMT is a highly conserved embryonic morphogenesis program that converts epithelial cells to migratory mesenchymal cells. During embryogenesis, EMT is normally activated in a stem cell population called the Neural Crest (NC) that invades distant organs to form peripheral neurons and glia. Aberrant activation of EMT is a common feature of invasive tumors, so identifying the genetic and epigenetic mechanism controlling EMT is expected to reveal new therapeutic targets for pediatric cancer patients. Projects in this area include i) identifying the epigenetic and transcriptional regulators of EMT using Chromatin Immunoprecipitation and RNA Sequencing (ChIP-Seq) in zebrafish mutants and ii) generating genetic mutants in recently identified NC EMT effector molecules using CrispR/Cas9 technology to determine if they are required alone, or in combination, to execute NC EMT.
- Identifying embryonic mechanisms driving tumor invasion and chemoresistance. To directly test if the embryonic mechanisms identified during NC EMT impact brain tumor pathogenesis, we generate brain tumors in different NC mutant backgrounds and analyze tumor cell behaviors at the sub-cellular level using live imaging approaches. We have also developed a novel and highly scalable transplantation technique to grow human brain tumor cells in zebrafish embryonic brains to accelerate drug discovery and host/immune cell interactions. Current projects in the lab include analyzing the impact of inhibiting actin remodeling proteins and chemokine receptors, such as Cxcr4, in brain tumor invasion, metastasis and chemo/radiation-resistance.
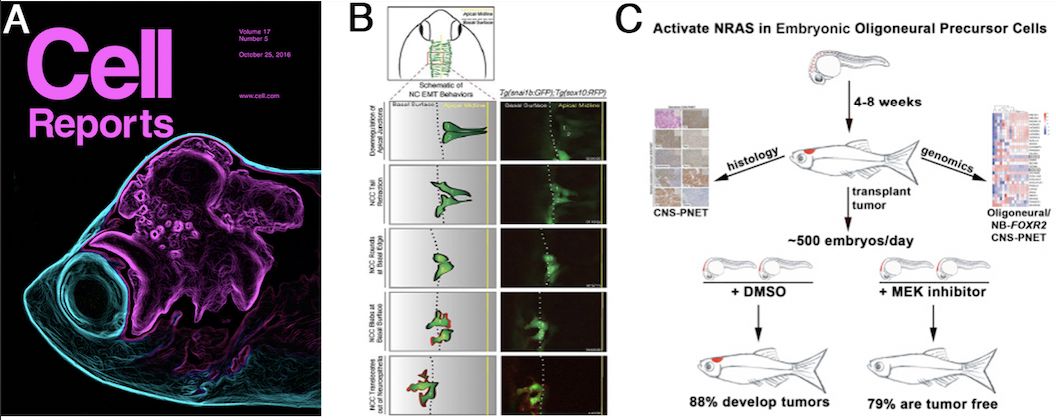
Figures:(A) Zebrafish pediatric brain tumor models. The transparent attribute of zebrafish allows real time imaging of brain tumor growth and invasion for genetic and drug screening. (B) Live imaging approaches identify NC cells undergoing EMT. An EMT reporter strain is used to identify new genetic and epigenetic mechanisms controlling EMT. (C) Overview of drug screening for new brain cancer therapeutics. We developed a scalable transplantation assay to grow fish or human tumor cells in the embryonic zebrafish brain to allow screening of drugs that reverse tumor growth.
References
- Pediatric Cancer Models in Zebrafish. Trends Cancer. 2020 May;6(5):407-418. doi: 10.1016/j.trecan.2020.02.006. Epub 2020 Mar 13. Review. PubMed PMID: 32348736; PubMed Central PMCID: PMC7194396.
- Sorrells S, Nik S, Casey M, Cameron RC, Truong H, Toruno C, Gulfo M, Lowe A, Jette C, Stewart RA#, Bowman TV# (2018). Spliceosomal components protect embryonic neurons from R-loop-mediated DNA damage and apoptosis. Dis Model Mech. PMID: 29419415.
- Casey MJ, Modzelewska K, Anderson D, Goodman J, Boer EF, Jimenez J, Grossman D and Stewart RA (2017). Transplantation of Zebrafish Pediatric Brain Tumors into Immune-competent Hosts for Long-term Study of Tumor Cell Behavior and Drug Response. J Vis Exp, (123). PMID: 28570545
- Casey MJ and Stewart RA (2017). Zebrafish as a model to study neuroblastoma development. Cell and Tissue Research. 372(2):223-232. PMID: 29027617
- Boer EF, Jette CA and Stewart RA (2016). Neural crest migration and survival are susceptible to morpholino-induced artifacts. PLoS ONE. 11(12):e0167278.
- Modzelewska K, Boer EF, Mosbruger TL, Picard D, Anderson D, Miles RR, Kroll M, Oslund W, Pysher TJ, Schiffman JD, Jensen R, Jette CA, Huang A, Stewart RA (2016). MEK Inhibitors reverse growth of embryonal brain tumors derived from oligoneural precursor cells. Cell Rep. 2016 Oct 25;17(5):1255-1264.
- Morrison MA, Zimmerman MW, Look AT, Stewart RA (2016). Studying the peripheral sympathetic nervous system and neuroblastoma in Methods Cell Biol. 2016;134:97-138.
- Jimenez L, Wang J, Morrison MA, Whatcott C, Soh KK, Warner S, Bearrs D, Jette CA and Stewart RA (2016). Phenotypic chemical screening using a zebrafish neural crest EMT reporter identifies retinoic acid as an inhibitor of epithelial morphogenesis. Dis Model Mech. 100: 127-52.
- Boer EF, Howell ED, Schilling TF, Jette CA, Stewart RA (2015). Fascin1-dependent Filopodia are required for directional migration of a subset of neural crest cells. PLoS Genet, 11(1), e1004946
