Suzanne L. Mansour
Professor of Human Genetics and Adjunct Professor of Neurobiology
FGFs and Inner Ear Development, Mouse Models of Hearing Loss and Restoration

Molecular Biology Program
Education
B.A. Harvard University
Ph.D. University of California, Berkeley
Research
The inner ear, which mediates the sensations of hearing and balance, is derived almost entirely from the otic placode, a small patch of ectodermal cells that is specified for an otic fate early in fetal development. Through a series of tissue interactions that are mediated by secreted signaling molecules, otic cells undertake complex processes of morphogenesis and differentiation to achieve their final functional form. Abnormalities of these processes lead to congenital deafness, which is the most common human sensory disorder. To better understand these disorders, my laboratory employs genetic and molecular approaches to identify and characterize genes that are important for the development and/or function of the mouse inner ear.
Fibroblast growth factor (FGF) signaling plays critical roles in the early development of the inner ear. Disruption of either Fgf3 or Fgf10, which encode ligands that signal through the same FGF receptor isoforms, FGFR1b and FGFR2b, leads to defects of mouse inner ear morphogenesis. Fgf3 is required from the adjacent hindbrain to direct dorsal otic gene expression and induction of the endolymphatic duct. Fgf3 null mutants develop a cystic inner ear and exhibit both hearing and vestibular dysfunction. Fgf10 is required for both posterior semicircular canal development and differentiation of cochlear non-sensory tissues. Fgf3/Fgf10 double null mutants have no ear development at all, showing that these genes are required redundantly for the initial induction of the otic placode. We used conditional mutants to dissect the tissue origins of these inductive FGF signals. In addition, microarray comparisons of control and FGF-deficient otic placodes revealed transcriptional targets of FGF signaling that are required for subsequent development of the inner ear.
Inactivation of Fgf3 and Fgf10 alleles specifically within the otic epithelium and induction of a ubiquitously secreted, dominant-negative (dn) form of FGFR2b, revealed dosage sensitive roles for these genes in directing otic neurogenesis as well as in both cochlear and vestibular morphogenesis. Differential RNA-seq analysis of dissected otic epithelium from control and dnFGFR2b embryos reveal novel up and down-regulated target genes that are the subject of current functional studies as potential hearing loss genes (Urness et al., 2018 BioRxiv 430876 preprint).
Many forms of sensorineural hearing loss, whether caused by genetic or environmental factors, are characterized by loss of cochlear sensory cells, which do not regenerate in mammals. In contrast, birds and fish regenerate lost sensory cells from residual cochlear supporting cells. Strategies for hearing restoration in mammals are focused on manipulating developmental signals to change cochlear supporting cell identity. We showed that mice with an activating mutation in FGFR3 that models Muenke syndrome have hearing loss associated with an identity switch between two types of supporting cells. Muenke syndrome model mice also have ectopic sensory cells. We found that supporting cell identity and hearing are restored in these FGFR3 mutants by genetically reducing the level of FGF10, a ligand does not normally activate FGFR3. Interestingly, the ectopic sensory cells remain. We went on to show that the Muenke syndrome mutation changes FGFR3 specificity so that it becomes responsive to FGF10. Most remarkably, we found that the supporting cell fate switch is actually completed the “rescued” animals, but is then gradually reversed. This shows that seemingly fully differentiated cochlear supporting cells can reversibly switch fates in an FGF-dependent manner. Since some immature supporting cells can be induced under certain circumstances to convert to sensory cells, this FGF-dependent plasticity is being investigated so that it can be exploited as a component of strategies designed to promote
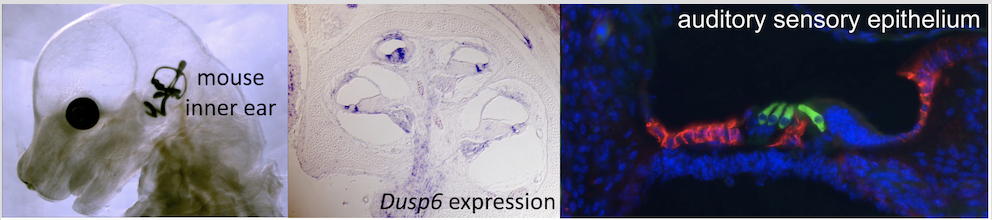
Fig. 1. (A) An E15.5 mouse embryo was cleared and its inner ear filled with latex paint. (B) Section taken through an E18.5 mouse cochlear duct showing expression of Dusp6 (purple). Cochlear duct section stained with antibodies directed against MYO7A (green) and CD44 (red) reveals sensory hair cells and supporting cells in the mouse inner ear.
References
-
Urness LD, Wang X, Doan H, Shumway N, Noyes CA, Gutierrez-Magana E, Lu R, Mansour SL (2018) Spatial and temporal inhibition of FGFR2b ligands reveals continuous requirements and novel targets in mouse inner ear morphogenesis. Development. 2018 Nov 30. pii: dev.170142. doi: 10.1242/dev.170142. [Epub ahead of print]
-
Quadros, RM, Miura, H, Harms, DW, Akatsuka, H, Sato, T, Aida, T, Redder, R, Richardson, GP, Inagaki, Y, Sakai, D, Buckley, SM, Seshacharyulu, P, Batra, SK, Behlke, MA, Zeiner, SA, Jacobi, AM, Izu, Y, Thoreson, WB, Urness, LD, Mansour, SL, Ohtsuka, M, Gurumurthy, CB (2017) Easi-CRISPR: a robust method for one-step generation of mice carrying conditional and insertion alleles using long ssDNA donors and CRISPR ribonucleoproteins. Genome Biol 18, 92.
-
Urness, LD, Wang, X, Shibata, S, Ohyama T and Mansour SL (2015) Fgf10 is required for specification of non-sensory regions of the cochlear epithelium. Dev. Biol., 400: 59-71
-
Mansour SL, Li C, Urness LD (2013) Genetic rescue of Muenke syndrome model hearing loss reveals prolonged FGF-dependent plasticity in cochlear supporting cell fates. Genes Dev.27: 2320-2331
-
Urness LD,, Paxton C,, Wang X, Schoenwolf GC,, Mansour SL (2010) FGF signaling regulates otic placode induction and refinement by controlling both ectodermal target genes and hindbrain Wnt8a. Dev. Biol. 340:595-604
-
Mansour SL, Twigg, SRF, Freeland RM, Wall SA, Li C, Wilkie AOM (2009) Hearing loss in a mouse model of Muenke syndrome. Hum. Mol. Genet. 18:43-50
-
Hatch E, Noyes CA, Wang X, Wright TJ and Mansour SL (2007) Fgf3 is required for dorsal patterning and morphogenesis of the inner ear epithelium. Development 134:3615-3625
-
Li C, Scott DA, Hatch E, Tian X, Mansour SL (2007) Dusp6 (Mkp3) is a negative feedback regulator of FGF-stimulated ERK signaling during mouse development. Development 134:167-176
-
Ladher RK, Wright TJ, Moon AM, Mansour SL, Schoenwolf GC (2005) FGF8 initiates inner ear induction. Genes Dev. 19:603-613
-
Wright TJ, Mansour SL (2003) Fgf3 and Fgf10 are required for mouse otic placode induction. Development 130:3379-3390
