John F. Atkins
Emeritus Research Professor of Human Genetics
Reprogrammed Decoding / Ribosome

Molecular Biology Program
Education
B.A. Dublin University
Ph.D. Dublin University
Sc.D. Dublin University
Research
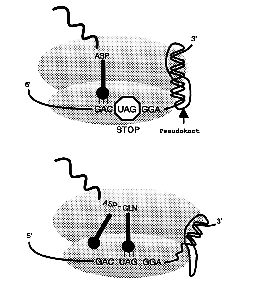
Codes with THE code. Signals buried within the coding sequence of a minority of mRNAs in probably all organisms stimulate a non-standard decoding event utilized for gene expression purposes. The non-standard event can be local redefinition of codon meaning, a shift of reading register or the bypassing of a block of nucleotides present in the mature mRNA. The efficiency of some of the "recoding" events is responsive to the cellular level of a component, such as polyamines or release factor 2, and these events can serve an autoregulatory function. In others there is a fixed ratio of the product of standard decoding to that of the recoding product which serves some unique function. We are investigating the occurrence, mechanisms involved and the function of recoding.
Our recent work has focused substantially on novel viral occurrences of recoding – it is especially prevalent in viral and chromosomal mobile element decoding. However, we are now concentrating more on utilization of recoding in chromosomal gene decoding. Particular mRNA structures 3' of the recoding site are well known as stimulators of recoding, though in one case we are currently investigating, intra-mRNA structure does not seem to be involved – in this we are investigating the possibility of mRNA in the ribosome entrance tunnel pairing with ribosomal RNA. Nevertheless, most of our current effort on stimulators is the interaction of nascent peptide sequence while still within the ribosome, interacting with ribosomal components of the peptide exit tunnel to mediate recoding. Also in a case of translational bypassing we have evidence for mRNA structure formation within the ribosome.
One fascinating case of codon redefinition is how the 10 UGA codons within human selenoprotein P specifies the 21st amino acid selenocysteine rather than termination. In collaboration with Dr. Mike Howard and others we are investigating mice which have mutants in the recoding signals involved.
References
- A.M. Hudson, G. Loughran, N.L. Szabo, N.M. Wills, J.F. Atkins, L. Cooley. 2021. Tissue specific dynamic codon redefinition in Drosophila. PNAS 118: e2012793118
- P.R. Bhatt, (11 others) J.F. Atkins, N. Ban. 2021. Structural basis of ribosomal frameshifting during translation of the SARS-CoV-2 RNA genome. Science 372, 1306-1313.
- Y.A. Khan, (6 others) J.S. Kieft, A.E. Firth, J.F. Atkins. 2021. Evaluating programmed frameshifting in CCR5 mRNA decoding. Nature in press.
- J.F. Atkins, K.M. O’Connor, P.R. Bhatt, G. Loughran. 2021. From Recoding to Peptides for MHC class I immune display: Enriching viral expression, virus vulnerability and virus evasion. Viruses 13, 1251.
- J. Choi, S. O’Loughlin, J.F. Atkins and J.D. Puglisi. 2020. The energy landscape of -1 ribosomal frameshifting. Sci. Adv. 6: eaax6969.
- S. O’Loughlin ..(others) ... and J.F. Atkins. 2020. Polysomes bypass a 50 nucleotide coding gap less 2 efficiently than monosomes due to attenuation of a 5’ mRNA stem loop and enhanced drop-off. J. Mol. Biol. 432, 4369-4387.
- T.R. Cech, J.A. Steitz and J.F. Atkins. 2019. eds. RNA Worlds: New Tools for Deep Exploration. (5th edition RNA Worlds books). 559 pages. Cold Spring Harbor Laboratory Press, New York. In print and e-book. Individual articles also Cold Spring Harbor Perspectives in Biology.
- J. Baclaocos ..... others) ... and J.F. Atkins, J.F. 2019. Up to 132 UGAs in metazoan selenoprotein P mRNAs: Regulatory encoding of selenocysteine in oyster. J. Mol. Biol.431, 4381-4407.
- C. Penno, R. Kumari, P.V. Baranov, D. van Sinderen and J.F. Atkins. 2017. Specific Reverse Transcriptase slippage at the HIV ribosomal frameshift sequence: Potential implications for modulation of GagPol synthesis. Nucl. Acids Res. In press.
- C. Penno, R. Kumari, P.V. Baranov, D. van Sinderen and J.F. Atkins. 2017. Stimulation of reverse transcriptase generated cDNAs with specific indels by template RNA structure: Retrotransposon, dNTP balance, RT-reagent usage. Nucl. Acids Res. in press.
- I.P. Ivanov, J. Wei, S.Z. Caster, K.M. Smith, A.M. Michel, Y. Zhang, A.E. Firth, M. Freitag, J.C. Dunlap, D. Bell-Pedersen, J.F. Atkins and M.S. Sachs. 2017. Translation initiation from conserved non-AUG codons provides additional layers of regulation and coding capacity. mBio 8. pii: e00844-17.
- G. Loughran, M.T. Howard, A.E. Firth and J.F. Atkins. 2017. Avoidance of reporter assay distortions from fused dual reporters. RNA23, 1285- 1289.
- J.F. Atkins, G. Loughran and P.V. Baranov. 2017. A [Cu]rious ribosomal profiling pattern leads to the discovery of ribosomal frameshifting in the synthesis of a copper chaperone. Mol. Cell65, 203-204.
- A.V. Lobanov, S.M. Heaphy, A.A. Turanov, M.V. Gerashchenko, S. Pucciarelli, R.R. Devaraj, F. Xie, V.A. Petyuk, R.D. Smith, L.A. Klobutcher, J.F. Atkins, C. Miceli, D.L. Hatfield, P.V. Baranov and V.N. Gladyshev. 2017. Position-dependent termination and widespread obligatory frameshifting in Euplotes translation Nature Struct. Mol. Biol. 24, 61-68.
- S. Wu, M. Mariotti, D. Santesmasses, K.E. Hill, J. Baclaocos, E. Aparicio-Prat, S. Li, J. Mackrill, Y. Wu, M.T. Howard, M. Capecchi, R. Guigó, R.F. Burk and J.F. Atkins. 2016. Human selenoprotein P and S variant mRNAs with different numbers of SECIS elements and inferences from mutant mice of roles of multiple SECIS elements. Open Biology 6.pii:160241. PMID: 27881738.
- S.M. Heaphy, M. Mariotti, V.N. Gladyshev, J.F. Atkins and P.V. Baranov. 2016. Systematic evaluation of the genetic code in ciliates reveals reassignment of all three stop codons to sense codons in Condylostoma magnum. Mol. Biol. Evol. 33, 2885-2889.
- P. Saffert, F. Adamla, R. Schieweck, J.F. Atkins and Z. Ignatova. 2016. An expanded CAG repeat in huntingtin causes +1 frameshifting. J. Biol. Chem. 291, 18505-18513.
- J.F. Atkins, G. Loughran, P.R. Bhatt, A.E. Firth and P.V. Baranov. 2016. Ribosomal frameshifting and transcriptional slippage: From genetic steganography & cryptography to adventitious use. Nucl. Acids Res. 44, 7007-7078.
- I. Tzani, I.P. Ivanov, D.E. Andreev, K.A. Dean, P.V. Baranov, J.F. Atkins and G. Loughran. 2016. Systematic analysis of the PTEN 5' leader identifies a major AUU initiated proteoform. Open Biol. 6.pii:150203.
- J. Chen, A. Coakley, M. O’Connor, A. Petrov, S.E. O’Leary, J.F. Atkins and J.D. Puglisi. 2015. Coupling of mRNA structure rearrangement to ribosome movement during bypassing of non-coding regions. Cell163, 1267-1280.
- P.V. Baranov, J.F. Atkins and M.M. Yordanova. 2015. Augmented Genetic Decoding: global, local and temporary alterations of decoding processes and codon meaning. Nature Rev. Genet. 16, 517-29.
- M.M. Yordanova, C. Wu, D.E. Andreev, M. Sachs, J.F. Atkins. 2015. A nascent peptide signal responsive to physiological levels of polyamines acts to stimulate regulatory frameshifting on antizyme mRNA. J. Biol. Chem. 290, 17863–17878.
- A. Olspert, B. Y.-W. Chung, J.F. Atkins, J. P. Carr and A. E. Firth. 2015. Transcriptional slippage in the positive sense RNA virus family Potyviridae. EMBO Rep. 16, 995-1004.
- C. Penno, V. Sharma, A. Coakley, M.O’C. Motherway, D. van Sinderen, L. Lubkowska, M.L. Kireeva, M. Kashlev, P.V. Baranov and J.F. Atkins. 2015. Productive mRNA stem-loop mediated transcriptional slippage: crucial features in common with intrinsic terminators. Proc. Natl. Acad. Sci. USA 112: E1984-1993.
