Kristen Kwan
Associate Professor of Human Genetics and Adjunct Associate Professor of Ophthalmology and Visual Sciences
Zebrafish, Eye Development and Morphogenesis

Molecular Biology Program
Education
B.S. Stanford University
Ph.D. Harvard University
Research
My lab studies the cellular and molecular mechanisms underlying tissue morphogenesis: the process by which a group of cells achieves its proper cellular organization and shape. Using the vertebrate eye as a model, we want to understand how the cells that comprise the vertebrate optic cup – neural retina, retinal pigmented epithelium, and lens – form the stereotyped structure that is critical for visual function. Developmental defects in eye morphogenesis represent a common cause of serious visual impairment in newborns.
Using confocal microscopy and a custom-built software suite for tracking cell behaviors in four dimensions, we have previously generated a map encompassing all cellular movements and divisions during zebrafish optic cup morphogenesis (Figure 1). We found that a complex set of cell movements, coordinated between different tissues, is responsible for shaping the eye. Using this unprecedented dataset as a reference point, our current studies focus on the molecular mechanisms underlying eye morphogenesis. Our studies make use of zebrafish molecular genetics, cell biology, and live imaging, and include the following projects:
Cell-matrix adhesion and cell polarity
We found that the extracellular matrix component laminin is required for eye morphogenesis: in bashfulUW1 (laminin-α1) mutants, optic stalk constriction and optic vesicle invagination are impaired (Figure 2). In addition, establishment of apicobasal polarity is impaired. However, we still do not understand how disrupted polarity leads to this phenotype, how polarity is initially established, or the signaling pathway downstream of laminin.
Developmental signaling pathways
While signaling pathways such as Shh and Wnt are known to play a role in patterning the optic vesicle, their role in morphogenesis itself is unknown. By performing 4-dimensional cell tracking on zebrafish mutants and morphants, we will quantitatively determine the contributions of different developmental signaling pathways to eye morphogenesis.
Cell motility
During eye morphogenesis, we find that retinal progenitors have unexpectedly motile behaviors despite their epithelial polarization. Such behaviors are frequently regulated by Rho-family small GTPases, and we will investigate the involvement of these signaling molecules in coordinating the motility of single cells and the morphogenesis of entire sheets if tissue.
New genes involved in eye morphogenesis
Our 4D analysis suggests that coordination of cell movements between tissues is critical for eye morphogenesis. In addition, in a forward genetic screen, I isolated a mutant, O15, in which extremely tight adhesion between the optic vesicle and the overlying ectoderm impairs invagination of both the optic vesicle and lens (Figure 2). We are isolating the underlying mutation and will determine the nature of the tissue-tissue interactions regulated by O15.
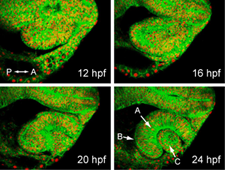
Timelapse imaging of zebrafish optic cup morphogenesis. Embryo is labeled uniformly for membranes (EGFP-CAAX) and nuclei (histone2A.F/Z-mCherry); single optical sections are shown from a 4D dataset. Eye morphogenesis occurs rapidly, between 12 and 24 hours post fertilization (hpf). At 24 hpf, the optic cup has three component tissues: (A) neural retina, (B) retinal pigmented epithelium, and (C) lens.
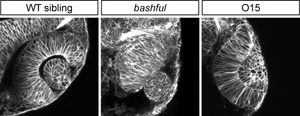
Distinctive eye morphogenesis phenotypes can be obtained in zebrafish. bashful (laminin-α1) mutant shows small eye and protruding lens. O15 (mutation currently being mapped) has incomplete invagination; note appearance of extremely tight adhesion between the retina and lens.
References
- Lusk S, Casey MA, and Kwan KM (2021). 4-dimensional imaging of zebrafish optic cup morphogenesis. J Vis Exp doi: 10.3791/62155. PMID: 34125104.
- Casey MA, Lusk S, and Kwan KM (2021). Build me up optic cup: Intrinsic and extrinsic mechanisms of vertebrate eye morphogenesis [Review]. Dev Biol doi: 10.1016/j.ydbio.2021.03.023
- Bryan CD, Casey MA, Pfeiffer RL, Jones BW, and Kwan KM (2020). Optic cup morphogenesis requires neural crest-mediated basement membrane assembly. Development, 147: dev181420 doi: 10.1242/dev.181420
- Carney KR, Bryan CD, Gordon HB, and Kwan KM (2020). LongAxis: a MATLAB-based program for 3D quantitative analysis of epithelial cell shape and orientation. Dev Biol doi: 10.1016/j.ydbio.2019.09.016
- Gordon HB*, Lusk S*, Carney KR, Wirick EO, Murray BF, Kwan KM (2018). Hedgehog signaling regulates cell motility and optic fissure formation during vertebrate eye morphogenesis. Development, doi:10.1242/dev.165068
- Bryan CD, Chien CB, Kwan KM (2016) Loss of laminin alpha 1 results in multiple structural defects and divergent effects on adhesion during vertebrate optic cup morphogenesis. Dev Biol. Jun 20. pii: S0012-1606(16)30025-2
- Kwan KM*, Otsuna H* , Kidokoro H, Carney KR, Saijoh Y, Chien C-B (2012) A Complex Choreography of Cell Movements Shapes the Vertebrate Eye. Development 139:359-372 (selected for Faculty of 1000)
- Kwan KM (2010) 25 years on, Developmental Biology remains dynamic, competent, and instructive (book review). Developmental Dynamics 239:3506-7
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien C-B (2007) The Tol2kit: a multisite-gateway based construction kit for Tol2 transposon transgenesis constructs. Developmental Dynamics 236:3088-99
- Lowe CJ, Terasaki M, Wu M, Freeman Jr. RM, Runft L, Kwan KM, Haigo S, Aronowicz J, Lander E, Gruber C, Smith M, Kirschner MW, Gerhart J (2006) Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS Biology 4(9):e291 (selected for Faculty of 1000)
- Kwan KM, Kirschner MW (2005) A microtubule-binding Rho-GEF controls cell morphology during Xenopus convergent extension. Development 132: 4599-610 (selected for Faculty of 1000 and featured in Development’s “In this Issue”)
- Ivanovska I, Lee E, Kwan KM, Fenger DD, Orr-Weaver TL (2004) The Drosophila MOS ortholog is not essential for meiosis. Current Biology 14: 75-80 (selected for Faculty of 1000)
- Murtaugh LC, Stanger BZ, Kwan KM, Melton DA (2003) Notch signaling controls multiple steps of pancreatic differentiation. Proceedings of the National Academy of Sciences USA 100: 14920-5
- Kwan KM, Kirschner MW (2003) Xbra functions as a switch between cell migration and convergent extension in the Xenopus gastrula. Development 130:1961–72 (selected for Faculty of 1000 and featured in Development’s “In this Issue”)
