David W. Grainger
University Distinguished Professor of Molecular Pharmaceutics, University Distinguished Professor of Biomedical Engineering, Adjunct Professor of Chemistry, of Orthopaedics, of Dentistry
Biomaterials and Drug Delivery, Materials in Medicine, Nanomaterials Toxicity, Diagnostics, Cell Therapy

Biological Chemistry Program
Education
B.A. Dartmouth College
Ph.D. University of Utah
Research
Biomedical materials applications
The Grainger group is broadly interested in applications of materials in medicine, including surgical implants, drug delivery systems, diagnostic assays, regenerative medicine, biotechnology and infection. Research requires broad use of many tools and techniques to yield new knowledge about these complex systems. Materials synthesis, extensive analytical characterization, drug formulation, in vitro assays and in vivo testing often comprise a typical student research program.
Implant-centered infection
All implants pre-dispose all tissues to increased infection risk. Despite many materials designs, implant innovations and antimicrobial approaches continuing to address infection prophylaxis and infection treatments, few anti-infective approaches ever make it beyond preclinical testing to clinical use. Fewer still are commercialized for global use. Very few strategies actually can show much efficacy in vivo in human infections despite promising in vitro antimicrobial efficacy and even some translation to animal implant models. Lack of agreement or standardization of experimental protocols, a general lack of correlation between in vitro and in vivo preclinical results and lack of validation between in vivo preclinical implant infection models and clinical (human) results. The Grainger group is interested in understanding the host tissue response to implanted materials, how this compromises the local immune response around implants, and how this leads to infection of implants. Improved implant designs and release of antimicrobials as combination devices are common strategies.
Cell-derived therapeutics
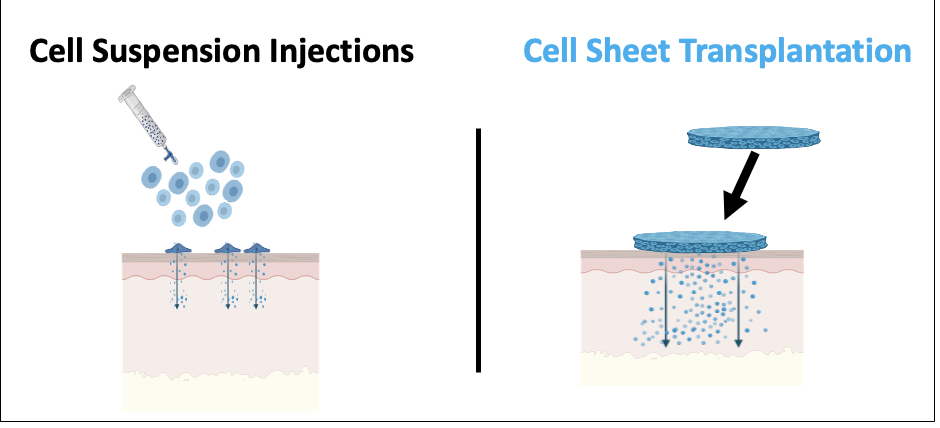
Over 900 clinical trials conducted using human mesenchymal stem cells in various diseases to date have produced little convincing therapeutic value and no FDA-approved products. Stem cell therapies in general show highly inconsistent outcomes in humans, distinct from their testing in animal disease models. We are interested in understanding this shortcoming and possible reasons for their poor human performance. We compare human cell therapies as injectable cell suspensions and surgically placed integral cell sheets for different behaviors and therapeutics markers in animal disease models. We are interested in translating cell sheets to specific human regenerative needs in kidney, cartilage and wound healing applications.
References
Implant-centered infection
- J. Busscher, V. Alt, H.C. van der Mei, P.H., Fagette, W. Zimmerli, T.F. Moriarty, J. Parvizi, G. Schmidmaier, M.J. Raschke, T. Gehrke, R. Bayston, L.M. Baddour, L.C. Winterton, R.O. Darouiche,D.W. Grainger, “A Trans-Atlantic Perspective on Stagnation in Clinical Translation of Antimicrobial Strategies for the Control of Biomaterial-Implant Associated Infection”, ACS Biomaterials Sci Eng, 2019, 5, 402−406.
- F. Moriarty, T.P. Schaer, G. Richards, H. Busscher, A.A. Montali, A. Moter, J. Wenke, H. Llinos, M.M. Riool, R. Mooney, S. Zeiter; V. Alt., N. Khanna, R. Kuehl, D.W. Grainger, “Recommendations for design and conduct of preclinical in vivo studies of orthopedic device-related infection, J. Orthoped. Res., 2019; 37(2),271-287; doi: 10.1002/jor.24230.
- Ren,P.H. Fagette, C.L. Hall, H. Broers, D.W. Grainger, H.C. van der Mei, H.J. Busscher, “Clinical translation of the assets of biomedical engineering - a retrospective analysis with looks to the future”, Exp. Rev. Med. Dev., (2019) https://doi.org/10.1080/17434440.2019.1685869.
- J. Busscher, W. Woudstra, T.G. van Kooten, P. Jutte, L. Shi, J. Liu, W.L.J. Hinrichs, H.W. Frijlink, R. Shi, J. Liu, J. Parvizi, S. Kates, V.M. Rotello, T.P. Schaer, D. Williams, D.W. Grainger, H.C. van der Mei, “Accepting higher morbidity in exchange for sacrificing fewer animals in studies developing novel infection-control strategies”, Biomaterials (2019); https://doi.org/10.1016/j.biomaterials.2019.119737
Cell sheet therapeutic technology
- Bou-Ghannam, K. Kim, M. Kondo, D.W. Grainger, T. Okano, Mesenchymal stem cell sheet centrifuge-assisted layering augments pro-regenerative cytokine production, Cells, 2022,11(18), 2840; https://doi.org/10.3390/cells11182840.
- M. Dunn, S. Kameishi, Y.Y. Cho, S.U. Song; D.W Grainger, T. Okano, Interferon-gamma primed human clonal mesenchymal stromal cell sheets exhibit enhanced immunosuppressive function, Cells(2022), 11(23), 3738; https://doi.org/10.3390/cells11233738.
- Oka, S. Kameishi, Y.K. Cho, S.U. Song, D.W. Grainger, T. Okano, Clinically relevant mesenchymal stem/stromal cell sheet transplantation method for kidney disease, Tissue Engineering C, Methods, (2023) 29(2) 54-62;doi: 10.1089/ten.TEC.2022.0200.
- Kameishi, M. Oka, C. Dunn, K-S Kim, Y.K. Cho, S.U. Song, D.W. Grainger, T. Okano, Rapid and effective preparation of clonal mesenchymal stromal cell sheets to reduce renal fibrosis, Sci. Rep., (2023) 13:4421; https://doi.org/10.1038/s41598-023-31437-7.
- M. Dunn, S. Kameishi, D.W. Grainger, T. Okano, Strategies to address mesenchymal stem/stromal cell heterogeneity in immunomodulatory profiles to improve cell-based therapies, Acta Biomaterialia, (2021) https://doi.org/10.1016/j.actbio.2021.03.069.
- Kondo, S. Kameishi, K.S. Kim, N.F. Metzler, T.G. Maak, D.T. Hutchinson, A.A. Wang, M. Maehara, M. Sato, D.W. Grainger, T. Okano, Safety and efficacy of human juvenile chondrocyte-derived cell sheets for osteochondral defect treatment. npj Regen Med, 6 65 (2021). https://doi.org/10.1038/s41536-021-00173-9.
- Thorp, K.S. Kim, T. Maak, D.W. Grainger, T. Okano, (2021) Enhancing Chondrogenic Potential via Mesenchymal Stem Cell Sheet Multilayering, Regen Med., 18, 487-496.